New paper in ACS Omega
10 June 2022
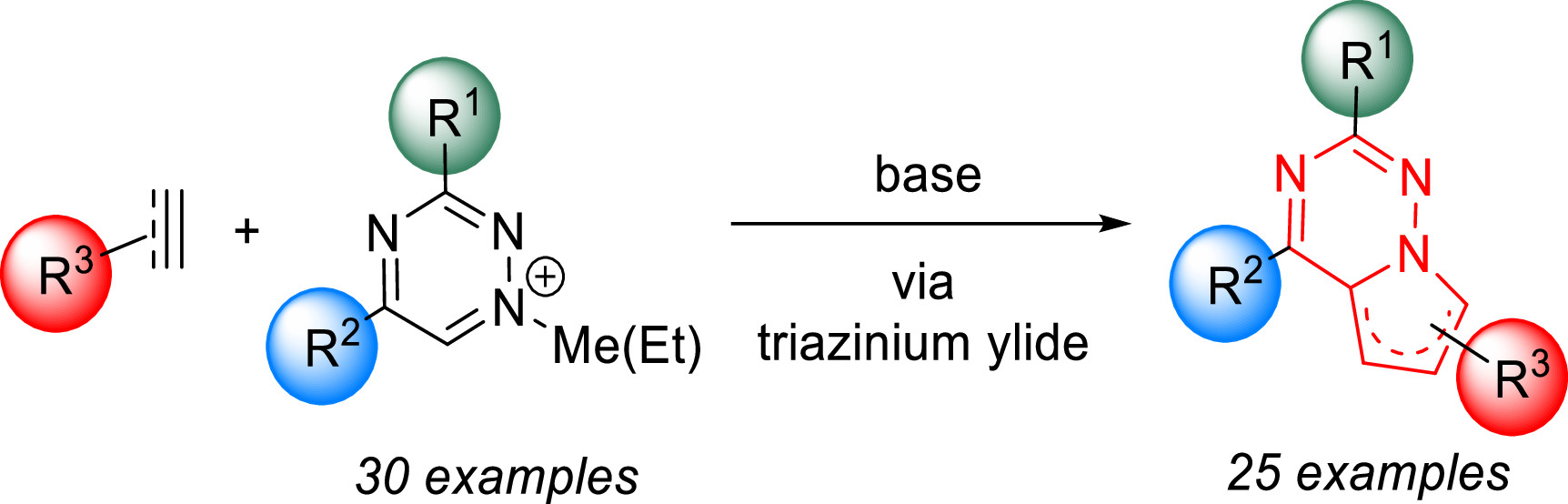
We published a new synthetic strategy to pyrrolo[2,1-f][1,2,4]triazines. We show that various synthetically easily accessible 1,2,4-triazines can be efficiently alkylated under mild conditions to provide the corresponding 1-alkyl-1,2,4-triazinium salts. These bench-stable salts serve as precursors to triazinium ylides, which react in 1,3-dipolar cycloadditions with electron-poor dipolarophiles to yield polysubstituted pyrrolotriazines in a single step.
- Galeta, J.; Šlachtová, V.; Dračínský, M.; Vrabel, M. Regio- and Diastereoselective 1,3-Dipolar Cycloadditions of 1,2,4-Triazin-1-ium Ylides: a Straightforward Synthetic Route to Polysubstituted Pyrrolo[2,1-f][1,2,4]triazines. ACS Omega 2022, 7, 21233-21238. https://doi.org/10.1021/acsomega.2c02276

