Blog
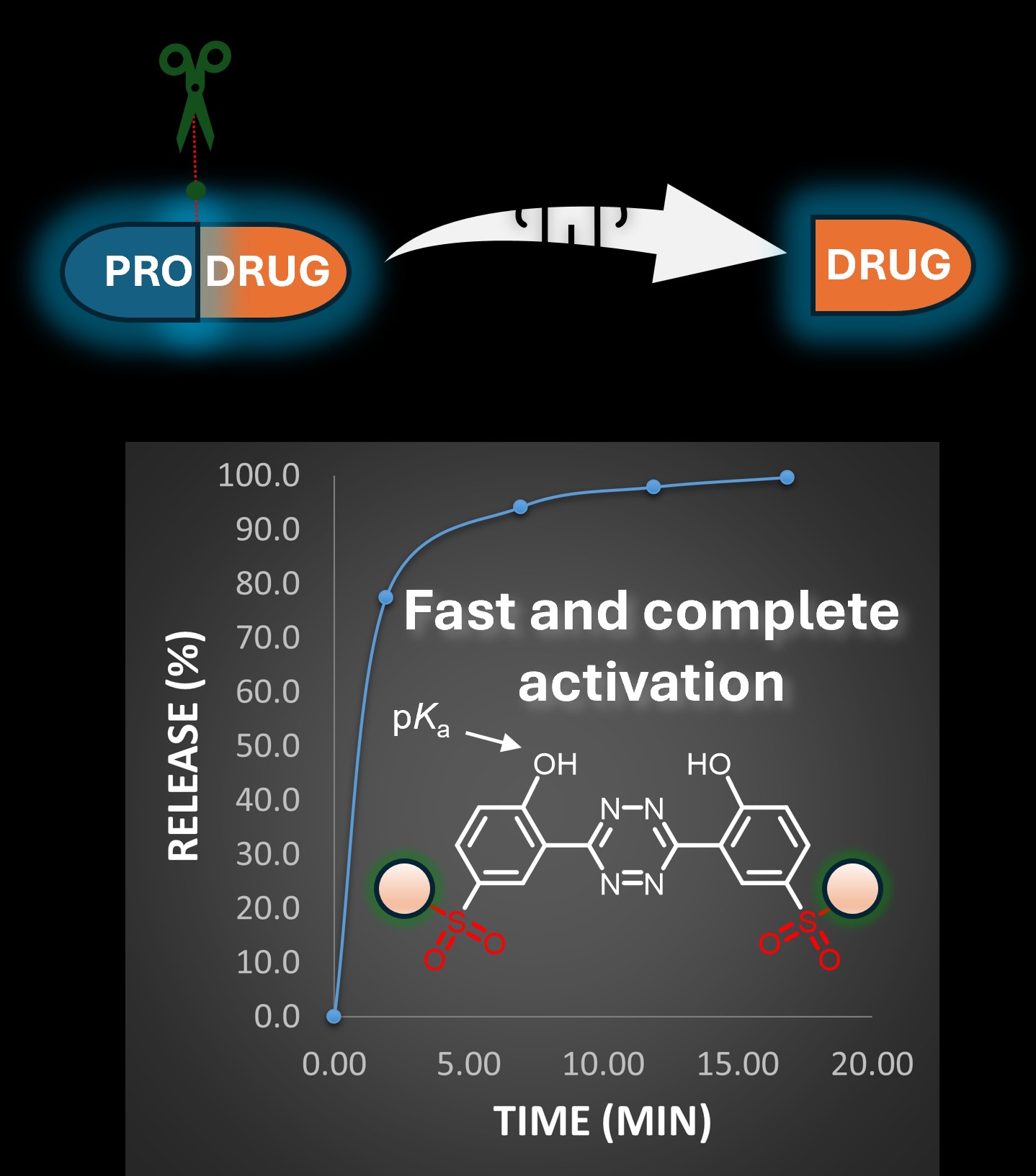
We have developed a new class of compounds capable of activating fluorophores or prodrugs at predetermined locations within living cells, enhancing both their precision and speed of action. One of the key advantages of these compounds is their ability to trigger fast and complete activation. This approach could, in the future, enable more targeted treatment of malignant tumors and significantly enhance the effectiveness of anti-cancer therapies. These next-generation prodrug activators are described in two recent papers published in Angewandte Chemie, in collaboration with the Mikula lab at TU Wien.
The articles:
- Rahm, M.; Keppel, P.; Dzijak, R.; Dračínský, M.; Šlachtová, V.; Bellová, S.; Reyes-Gutiérrez, P. E.; Štěpánová, S.; Raffler, J. E.; Tloušťová, E.; Mertlíková-Kaiserová, H.; Mikula, H.; Vrabel, M. Sulfonated Hydroxyaryl-Tetrazines with Increased pKa for Accelerated Bioorthogonal Click-to-Release Reactions in Cells. Angew. Chem. Int. Ed. 2024, e202411713. https://doi.org/10.1002/anie.202411713
- Wilkovitsch, M.; Kuba, W.; Keppel, P.; Sohr, B.; Löffler, A.; Kronister, S.; Fernandez del Castillo, A.; Goldeck, M.; Dzijak, R.; Rahm, M.; Vrabel, M.; Svatunek, D.; Carlson, J.; Mikula, H. Transforming Aryl-Tetrazines into Bioorthogonal Scissors for Systematic Cleavage of trans-Cyclooctenes. Angew. Chem. Int. Ed. 2024, e202411707. https://doi.org/10.1002/anie.202411707
Press release by IOCB Prague: https://www.uochb.cz/en/news/652/new-prodrug-activation-method-may-enable-faster-and-more-effective-treatment-of-severe-diseases
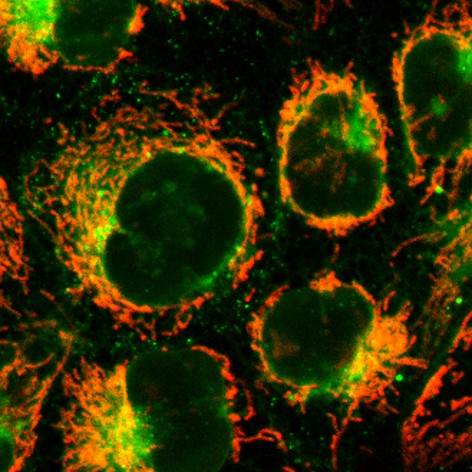
We have developed a new type of bioorthogonal — or biocompatible — chemical reaction that can proceed selectively under demanding biological conditions without causing harm. This innovative chemistry holds great promise for advancing the visualization and study of drug distribution in living cells, including within cancerous tumors. The reaction, known as triazinium ligation, enables the modification of biomolecules such as proteins and peptides, allowing their properties to be fine-tuned or enhanced. The reagents developed by our team offer several key advantages: they are highly stable in cellular environments, react rapidly, and exhibit excellent solubility.
The article: Šlachtová, V.; Bellová, S.; La-Venia, A.; Galeta, J.; Dračínský, M.; Chalupský, K.; Dvořáková, A.; Mertlíková-Kaiserová, H.; Rukovanský, P.; Dzijak, R.; Vrabel, M. Triazinium Ligation: Bioorthogonal Reaction of N1-Alkyl 1,2,4-Triazinium Salts. Angew. Chem. Int. Ed. 2023, n/a, e202306828. https://doi.org/10.1002/anie.202306828
Press release by IOCB Prague: https://www.uochb.cz/en/news/526/new-chemical-reaction-will-allow-to-better-target-drugs-in-the-human-body-and-suppress-their-side-effects
Turn on English captions.
We are happy to announce that one of the compounds, previously developed in our lab for effective labeling of (bio)molecules in living cells is now commercially available from SigutLabs: https://sigutlabs.com/product/coumfluor-tz1-cas-2594424-82-3-2/.
See the original paper:
Galeta, J.; Dzijak, R.; Obořil, J.; Dračínský, M.; Vrabel, M. A Systematic Study of Coumarin–Tetrazine Light-Up Probes for Bioorthogonal Fluorescence Imaging. Chem. Eur. J. 2020, 26, 9945-9953. https://doi.org/10.1002/chem.202001290

Our study on structurally redesigned bioorthogonal reagents for mitochondria-specific prodrug activation has made it to A Special Virtual Issue Celebrating the 2022 Nobel Prize in Chemistry for the Development of Click Chemistry and Bioorthogonal Chemistry, curated by 2022 Nobel Prize laureate and ACS Central Science Editor-in-Chief Carolyn Bertozzi.
Check out the virtual issue: https://pubs.acs.org/page/vi/bioorthogonal-click-chemistry
Read our paper: Dzijak, R.; Galeta, J.; Vázquez, A.; Kozák, J.; Matoušová, M.; Fulka, H.; Dračínský, M.; Vrabel, M. Structurally Redesigned Bioorthogonal Reagents for Mitochondria-Specific Prodrug Activation. JACS Au 2021, 1, 23–30. https://doi.org/10.1021/jacsau.0c00053
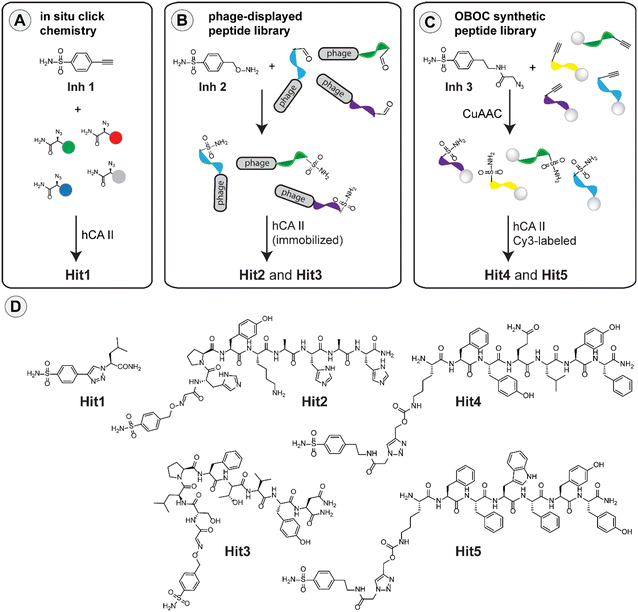
In collaboration with the Structural Biology Group, we have published a new article on the identification of specific carbonic anhydrase inhibitors via in situ click chemistry, phage-display, and synthetic peptide libraries.
The development of highly active and selective enzyme inhibitors is one of the priorities of medicinal chemistry. Typically, various high-throughput screening methods are used to find lead compounds from a large pool of synthetic compounds, and these are further elaborated and structurally refined to achieve the desired properties. In an effort to streamline this complex and laborious process, new selection strategies based on different principles have recently emerged as an alternative.
In the article, we compare three such selection strategies with the aim of identifying potent and selective inhibitors of human carbonic anhydrase II. All three approaches, in situ click chemistry, phage-display libraries, and synthetic peptide libraries, led to the identification of more potent inhibitors when compared to the parent compounds.
In addition, one of the inhibitor–peptide conjugates identified from the phage libraries showed greater than 100-fold selectivity for the enzyme isoform used for the compound selection. In an effort to rationalize the binding properties of the conjugates, we performed detailed crystallographic and NMR structural analysis, which revealed the structural basis of the compound affinity towards the enzyme and led to the identification of a novel exosite that could be utilized in the development of isoform specific inhibitors.
- Kugler, M.; Hadzima, M.; Dzijak, R.; Rampmaier, R.; Srb, P.; Vrzal, L.; Voburka, Z.; Majer, P.; Řezáčová, P.; Vrabel, M. Identification of specific carbonic anhydrase inhibitors via in situ click chemistry, phage-display and synthetic peptide libraries: comparison of the methods and structural study. RSC Medicinal Chemistry 2022. https://doi.org/10.1039/D2MD00330A
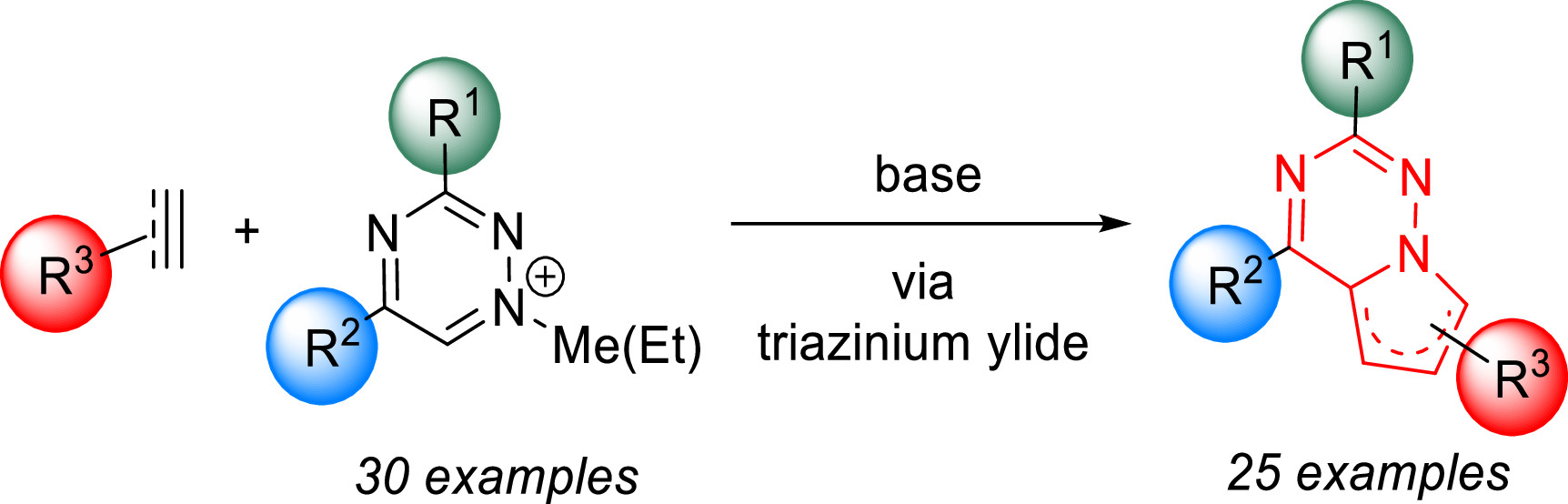
We published a new synthetic strategy to pyrrolo[2,1-f][1,2,4]triazines. We show that various synthetically easily accessible 1,2,4-triazines can be efficiently alkylated under mild conditions to provide the corresponding 1-alkyl-1,2,4-triazinium salts. These bench-stable salts serve as precursors to triazinium ylides, which react in 1,3-dipolar cycloadditions with electron-poor dipolarophiles to yield polysubstituted pyrrolotriazines in a single step.
- Galeta, J.; Šlachtová, V.; Dračínský, M.; Vrabel, M. Regio- and Diastereoselective 1,3-Dipolar Cycloadditions of 1,2,4-Triazin-1-ium Ylides: a Straightforward Synthetic Route to Polysubstituted Pyrrolo[2,1-f][1,2,4]triazines. ACS Omega 2022, 7, 21233-21238. https://doi.org/10.1021/acsomega.2c02276

We have won an ERC Proof of Concept Grant for the project CHEMCELL: Chemical Engineering of Natural Killer Cells for Cancer Immunotherapy, and 150,000 euros for the next 18 months to explore the commercial or societal potential of our discoveries within the SWEETOOLS project.
“Originally, we were interested in performing various harmless but beneficial chemical reactions on living cells, such as staining them with fluorescent tags in order to visualize them in real time in the living organism, which would be very useful for example in diagnostics. But then we discovered that we could do much more, like placing a molecule on a natural killer cell that can quickly direct it straight to a tumor cell to destroy it.
Thanks to this grant, we’ll be able to directly compare our method with CAR T-cell therapy and verify its real merit not only on cells but also in mice, which is a necessary step before use in human medicine. If the study goes well, our method could be adopted into practice in a matter of a few years,” says Milan.
Read more in the IOCB news: European grant opens the way for validation of a novel method for use in immunotherapy
Check out our chapter about the application of CuAAC reaction for peptide and protein modification written by Agustina, Anna, and Milan and published now within the Science of Synthesis series.
- La Venia, A.; Kovalová, A.; Vrabel, M. 2.3 CuAAC in Protein Conjugation. In Click Chemistry, 1st edition ed.; Georg Thieme Verlag KG: Stuttgart, 2022; Vol. 2021/4. https://doi.org/10.1055/sos-SD-235-00062
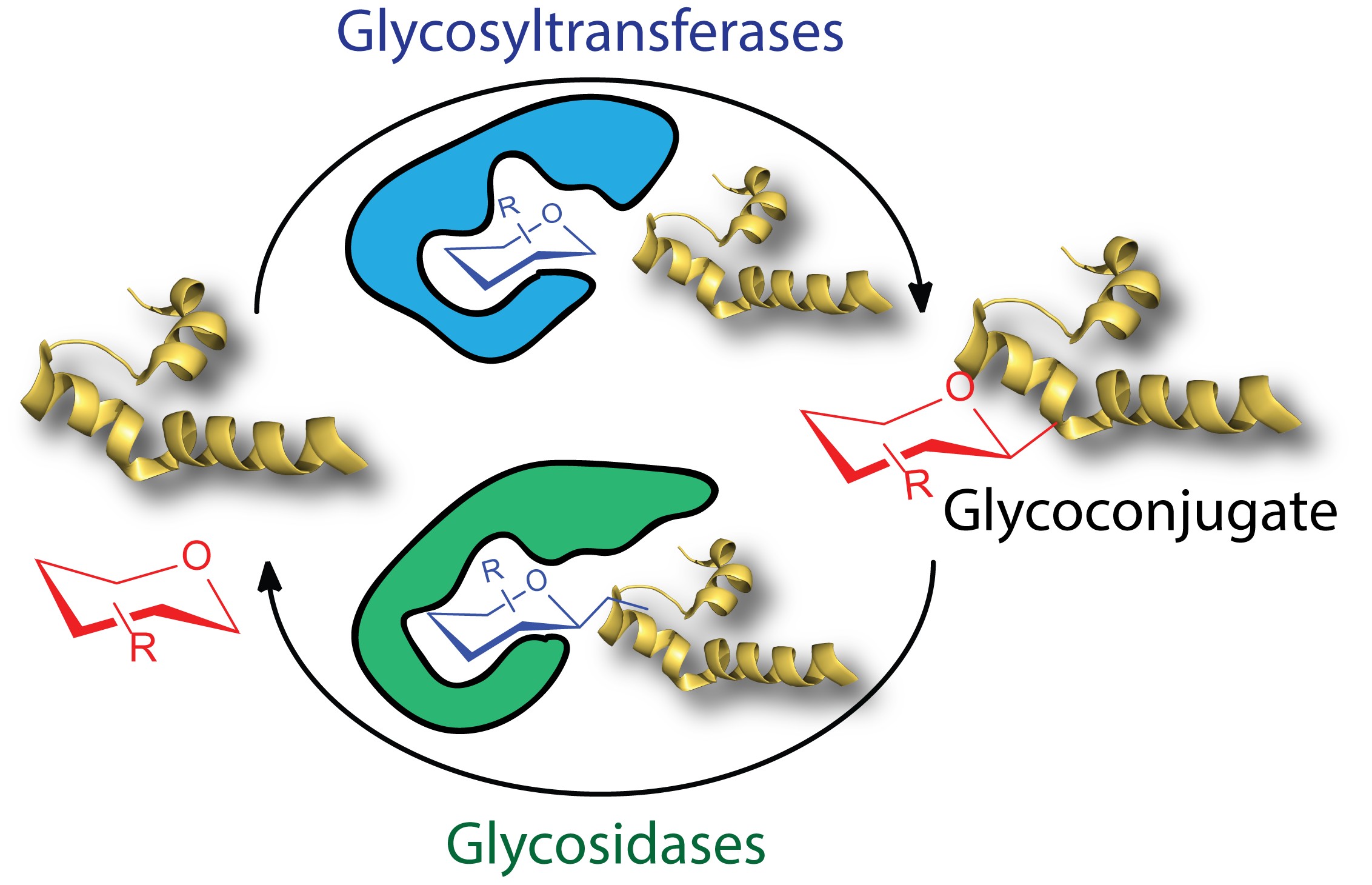
Read the main achievements of our work within the ERC-funded project SWEETOOLS which are now highlighted on the CORDIS website.
Our SWEETOOLS articles
Milan and Michal attended the 55th Advances in Organic, Bioorganic and Pharmaceutical Chemistry Conference (Liblice 2021), where Michal presented his work on a poster.
Great food, great location, great science and everything finally in person!

