Development of new bioorthogonal reagents
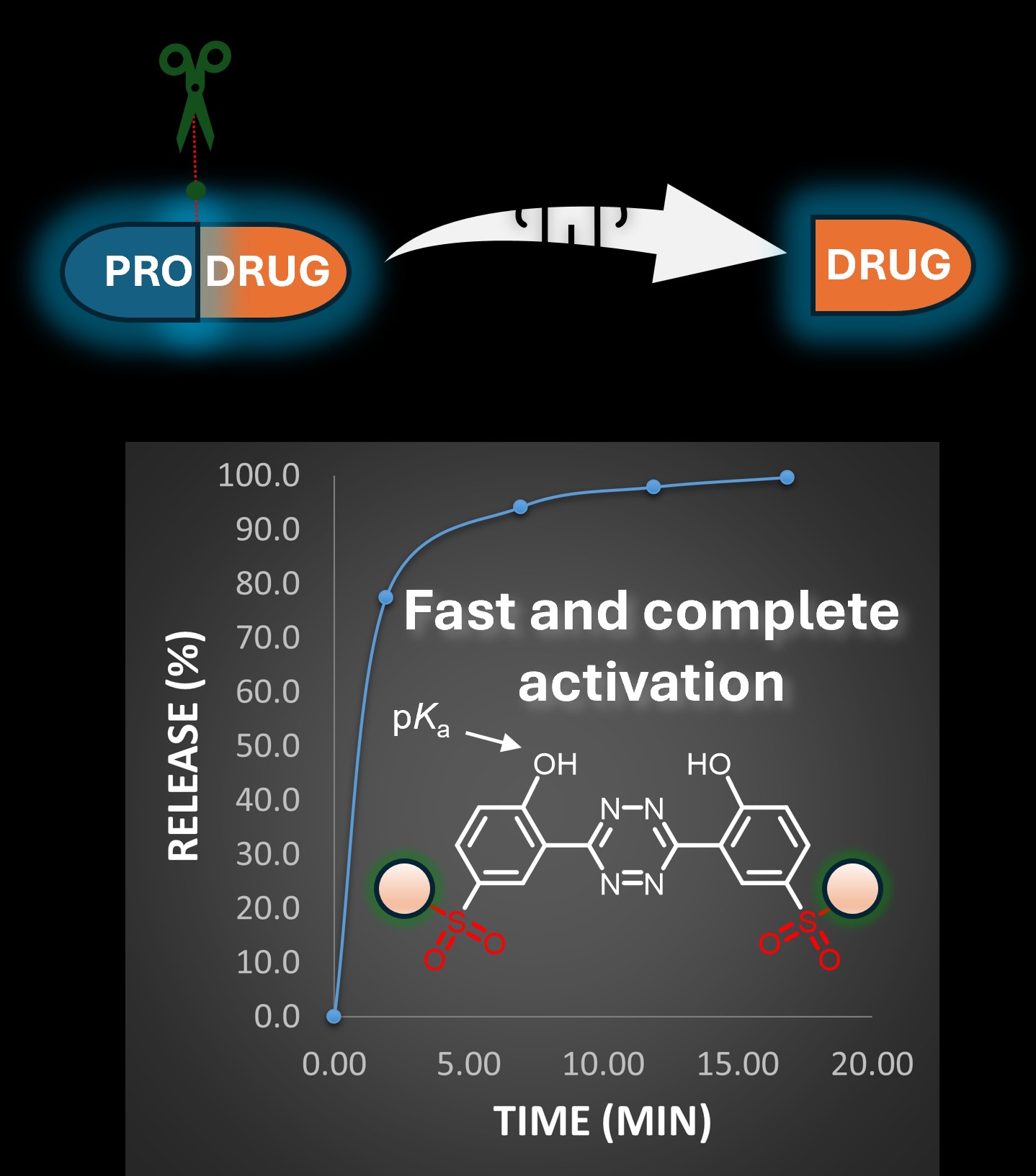
We have developed a new class of compounds capable of activating fluorophores or prodrugs at predetermined locations within living cells, enhancing both their precision and speed of action. One of the key advantages of these compounds is their ability to trigger fast and complete activation. This approach could, in the future, enable more targeted treatment of malignant tumors and significantly enhance the effectiveness of anti-cancer therapies. These next-generation prodrug activators are described in two recent papers published in Angewandte Chemie, in collaboration with the Mikula lab at TU Wien.
The articles:
- Rahm, M.; Keppel, P.; Dzijak, R.; Dračínský, M.; Šlachtová, V.; Bellová, S.; Reyes-Gutiérrez, P. E.; Štěpánová, S.; Raffler, J. E.; Tloušťová, E.; Mertlíková-Kaiserová, H.; Mikula, H.; Vrabel, M. Sulfonated Hydroxyaryl-Tetrazines with Increased pKa for Accelerated Bioorthogonal Click-to-Release Reactions in Cells. Angew. Chem. Int. Ed. 2024, e202411713. https://doi.org/10.1002/anie.202411713
- Wilkovitsch, M.; Kuba, W.; Keppel, P.; Sohr, B.; Löffler, A.; Kronister, S.; Fernandez del Castillo, A.; Goldeck, M.; Dzijak, R.; Rahm, M.; Vrabel, M.; Svatunek, D.; Carlson, J.; Mikula, H. Transforming Aryl-Tetrazines into Bioorthogonal Scissors for Systematic Cleavage of trans-Cyclooctenes. Angew. Chem. Int. Ed. 2024, e202411707. https://doi.org/10.1002/anie.202411707
Press release by IOCB Prague: https://www.uochb.cz/en/news/652/new-prodrug-activation-method-may-enable-faster-and-more-effective-treatment-of-severe-diseases

