New bioorthogonal reagents and reactions
Bioorthogonal chemical reactions have revolutionized modern chemical biology, greatly enhancing our capacity to precisely manipulate and label biomolecules in their native environment. Notably, the development of click and bioorthogonal reactions was recognized with the Nobel Prize in Chemistry in 2022, underscoring the great potential and influence of these techniques on modern chemical disciplines. Despite significant advancements in this field, there remains a pressing need for novel methods to overcome the limitations of existing technologies and reagents.
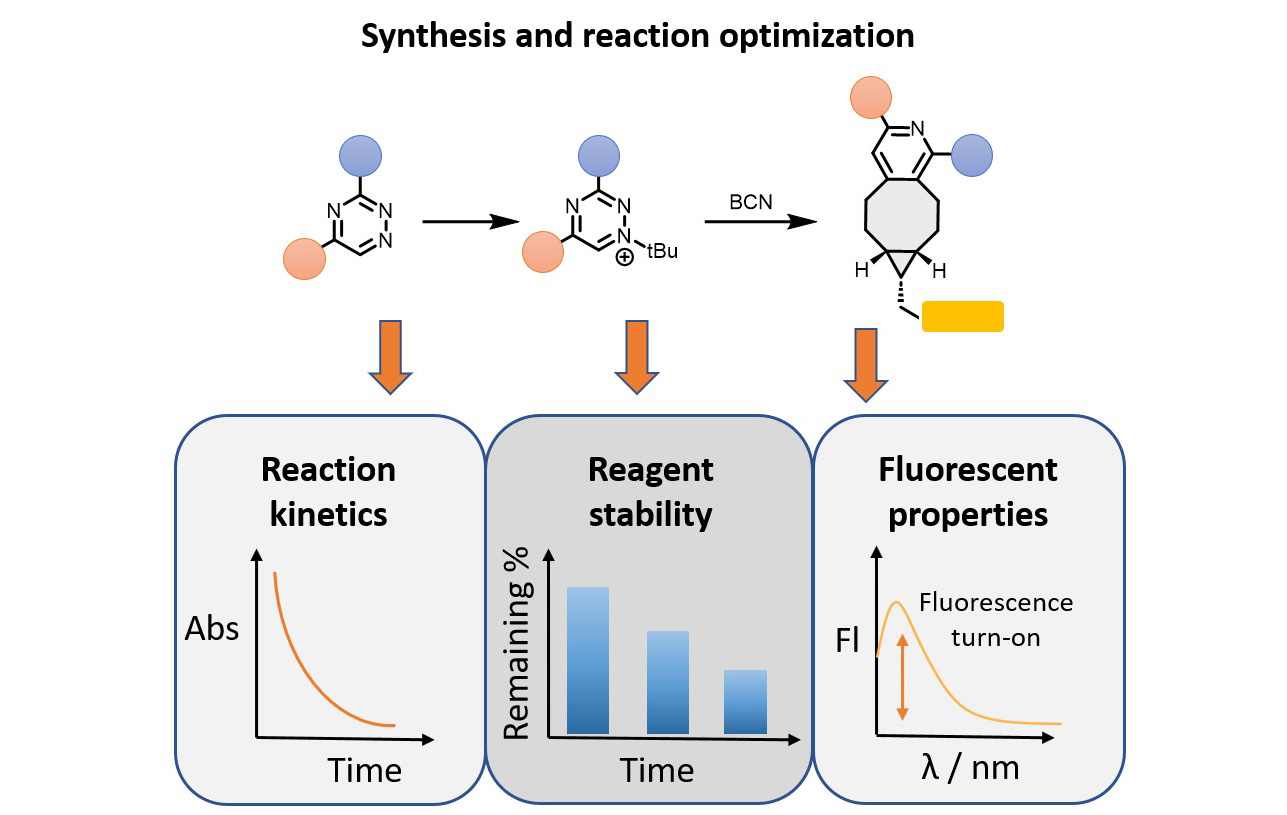
Our group is dedicated to improving existing bioorthogonal reagents and pioneering new reactions in the field. To achieve this, we integrate principles of synthetic organic chemistry with biological methodologies, providing a complex understanding of molecular characteristics and behavior. Following synthesis in the laboratory, our investigations involve measuring rate constants using techniques such as HPLC or UV/Vis spectroscopy to identify the most reactive derivatives. These derivatives are then further optimized for stability and selectivity under physiological conditions. In our approach, we frequently employ probes that yield fluorophores upon biocompatible reactions, facilitating elucidation and optimization of reactivity in cellular experiments by using fluorescence microscopy. We are interested in both, bioconjugation reactions and in bioorthogonal cleavage reactions.

